by Heinz U. Lemke, PhD
Part 1 from the last issue is available here.
Part 2 from the last issue is available here.
Part 3 from the last issue is available here.
Part 4: International approval issues
A critical question for the development of IT architectures and standards which support interoperability in the OR, relate to the issues they raise in risk assessment and the appropriate classification by the international approval processes for medical devices and systems.
In the following, the current situation of approval procedures in the USA and Japan will be briefly outlined (in a follow-up publication they will be compared to regulatory developments in Europe). Most of these observations are based on presentation and discussions in the course of a CARS 2014 DICOM in Surgery and IHE Surgery Workshop on “DICOM Supplements and IHE Integration Profiles, Implementation and Approval Issues” which took place in Fukuoka, Japan on June 28, 2014.
4.1 FDA (USA)
It appears that the FDA is taking an active role in the discussion relating to interoperability and corresponding issues in approval regulations by also being a member of the MD PnP project [1].
Support for MD PnP program work has come from DoD/TATRC, NSF, NIST, CIMIT, and NIH/NIBIB, which awarded a $10M Quantum grant in October 2010 to develop a healthcare intranet based on integrated medical device systems.
An important part of the key MD PnP Program projects is [2] “Defining a safe regulatory pathway for patient – centric networked medical devices.” This being carried out in close partnership with the FDA, progress so far includes a co-sponsored workshop held by FDA in January 2010 on medical device interoperability, followed by a working group of companies, academics, and hospitals that have developed and submitted a pre IDE (Investigational Device Exemption) regulatory submission to help refine the FDA clearance process. Some of the questions posed by representatives from FDA include [5]:
- Clinical issues What clinical scenarios could make use of medical device interoperability? Are there clinical scenarios that would not be appropriate?
- Engineering issues How should medical device interoperability be defined in terms of architecture, components, interfaces, functional requirements and performance requirements
- Risk issuesWhat are the risks associated with medical device interoperability and systems of systems composing medical devices? Use of risk models for interoperable systems.
- Management issues Who are the responsible parties and what is their role in design, building, maintenance, improvement as well as development and dissemination of standards and best practices.
It is interesting to note, that the FDA is responding positively to 510(k) application which include in their device description compliance to IHE, DICOM and HL7. For an example see [6] which refers to a recent PACS approval procedure by a major manufacturer who included in its device description:
“Centricity PACS is a standards-based, customizable, and scalable solution supporting several of the Integrating the Healthcare Enterprise (IHE) profiles, Digital Imaging and Communications in Medicine (DICOM), and the Health Level Seven (HL7) protocol standards for managing digital medical images and patient data. Centricity PACS supports radiographic imaging-as in clinical radiography, cardiology, dentistry, and mammography and non-radiologic imaging, including video support”.
Also in the area of PACS components or devices it can be observed that compliance to IHE integration profiles is thought to be a significant advantage in FDA approval procedures. For example, Three Palm Software, LLC stated in their application [7]:
“The enterprise workflow of the workstation (WorkstationOneTM Breast Imaging Workstation) follows IHE integration profiles, specifically, MAMMO (Mammography Image Profile) and RWP (Reporting Workflow Profile)”.
Another example of FDA approval applications with IHE integration profiles is in the area of digital radiography software tools for Quality Assessment (QA), in particular “Standardized Dose Reporting for QA” [8]. The Alliance for Radiation Safety in Pediatric Imaging recommends the IHE Radiation Exposure Monitoring (REM) profiles and DICOM Structured Reports (SR) to be applied in this context.
For the purpose of approval, medical devices and systems, such as given above need to be grouped into one of three FDA regulatory classes: Class I, II or III, depending upon the degree of regulation necessary to provide reasonable assurance of their safety and effectiveness. The three device classes are currently defined as follows [9]:
- Class I: Devices are subject to a comprehensive set of regulatory authorities called general controls that are applicable to all classes of devices.
- Class II: Devices for which general controls, by themselves, are insufficient to provide reasonable assurance of the safety and effectiveness of the device, and for which there is sufficient information to establish special controls to provide such assurance.
- Class III: Devices for which general controls, by themselves, are insufficient and for which there is insufficient information to establish special controls to provide reasonable assurance of the safety and effectiveness of the device. Class III devices typically require premarket approval.
Most medical devices can be classified by finding the matching description of the device in Title 21 of the Code of Federal Regulations (CFR), Parts 862-892. FDA has classified and described over 1,700 distinct types of devices and organized them in the CFR into medical specialty “panels” such as Cardiovascular devices or Ear, Nose, and Throat devices. The devices most relevant for the OR can be found in Part 878 entitled General and Plastic Surgery, Part 876 entitled Gastroenterology-Urology Devices and in Part 892 entitled Radiology. There exists an extremely comprehensive set of guidelines on how to apply for FDA Premarket Approval (PMA) or premarket notification (often referred to as a 510(k). This is particular the case also when there is software contained in medical devices [10]. An approval application is usually supported by a list of standards which the medical device/system has been shown in tests to be in compliance with. Most of the well known national and international standard bodies are explicitly recognized by the FDA. This list does not include IHE (as IHE is not a standardization organization) but examples of FDA approval application demonstrate that IHE compliance is being used in the device descriptions as a marker for quality. How the importance of compliance with IHE integration profiles is being rated in the approval process, however, is not made clear by the FDA guidelines for Industry as well as their own Food and Drug Administration Staff. A very special situation exists for an approval application for an IDE, relating to clinical trial approval by foreign companies. In this case, the sponsor of the clinical trial is responsible for submitting the IDE application to the FDA (§812.40) and obtaining Institutional Review Board (IRB) approval before the study can begin. Foreign companies wanting to conduct a clinical study in the U.S. MUST have a U.S. sponsor (§812.18).
4.2 PMDA (Japan)
The Pharmaceuticals and Medical Devices Agency (PMDA) is the FDA equivalent agency for approval procedures for medical and surgical devices and systems in Japan. In principle, it can be observed, that the medical device approval procedure is harmonized with those of other advanced countries.
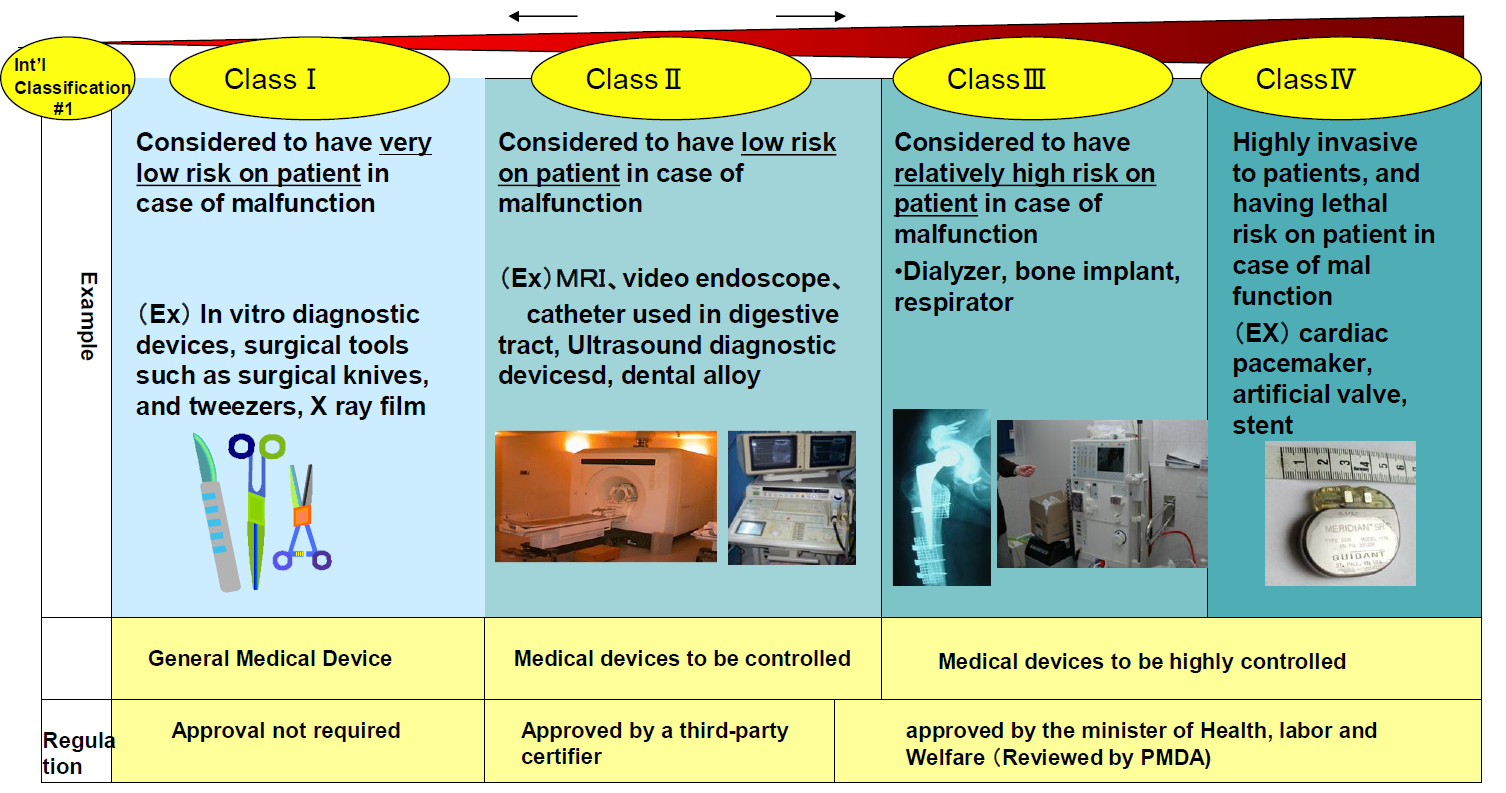
Figure 5 shows the classification used by PMDA, in principle derived from activities of the GHTF (Global Harmonization Task Force) (USA, EU, Australia, Canada, and Japan). It is (almost) in line with respect to the FDA classification, except that an extra Class IV has been added for highly risky devices. For Class II devices, third-party certifiers (in EU terminology: notified bodies) are approved by the Minister of Health, Labor and Welfare (MHLW). The approval criteria, however, are defined by MHLW. It is expected that after November 26, 2014 third-party certifiers will also be permitted to review and approve Class III devices.
As regards approval for software, it is important to note, that high risk health software running on non medical devices will be regulated after autumn, 2014. Specifically, software operated in non-medical devices (such as PC and tablet) used for high risk application will be reviewed by PMDA also after autumn, 2014. It can be expected, that the safety requirement defined in international standards will be referred to. Surgical navigation software running on conventional PC will also be regulated.
An important point of discussion in Japan relates also to the question whether the clinical data obtained in foreign countries is applicable to the review process in Japan. Specific issues are:
- clinical environment,
- differences in anatomy, pathology, depending on race, etc.,
- comparison with standard care.
In general, the PMDA profile of services as indicated in the 6 phases (top of Fig. 6), i.e. Research and development, Non-clinical tests, Clinical Test, Filing of application, Approval and Marketing, are similar to the FDA and European approval services. It is important to note, that standards development is considered to be a continuous activity in the PMDA profile of services. It is also recognized by PMDA that, in order to improve on the profile of services [11], a promotion of regulatory sciences is important to accelerate R&D of medical devices as well as an enhanced international cooperation. PMDA, therefore actively promotes international activities in line with the PMDA International Strategic Plan and the International Vision formulated in 2009 and 2011, and as well as a road map for more specific action plans defined in 2013. In order to build closer relationships with the EU and the US, PMDA has dispatched its staff members to regulatory agencies abroad including the European Medicines Agency. Moreover, PMDA’s ties with other regulators from the US, Europe, and Asia have been reinforced by means of holding PMDA training seminars and the exchange of trainees.
![Fig. 6. PMDA profile of services 2013-2014 [11]](https://news.iscas.co/wp-content/uploads/2016/03/p42-issue4-300x193.png)
References
[1] Goldman JM (2007): The OR of the Future: Current activities and Health IT implications, HIMSS07 New Orleans. http://www.mdpnp.org/uploads/HIMSS_2007_ORF_Goldman.pdf[2] http://www.mdpnp.org/about.html, accessed August 2014
[5] The FDA (CDRH) Workshop on Medical Device Interoperability: achieving safety and effectiveness, Co-Sponsored by The Continua Health Alliance and The Center For Integration Of Medicine & Innovative Technology (CIMIT), Medical Device “Plug-and-Play” Interoperability Program (MD PnP),
January 25-27, 2010
[6] www.accessdata.fda.gov/cdrh_docs/pdf11/k110875.pdf
[7] www.accessdata.fda.gov/cdrh_docs/pdf7/k073272.pdf 2008-01-08
[8] Don S, The Alliance for Radiation Safety in Pediatric Imaging, 2011, www.fda.gov/downloads/medicaldevices/newsevents/workshopsconferences/ucm313229.pdf
[9] The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notification, July 28, 2014
[10] General principles of software validation; Final guidance for industry and FDA staff, 2002
[11] PMDA profile of services 2013-2014.pdf
Heinz U. Lemke, PhD is a professor of Computer Science at the Technical University of Berlin since 1974, where he teaches and supervises research on the theme of Technical Informatics in Biomedicine. Since 1983 Heinz Lemke is the organizer of the congress series Computer Assisted Radiology and Surgery (CARS), editor-in-chief of the CARS Proceedings of the International Journal of CARS and executive director of the International Foundation of CARS.

Leave a Reply