by Heinz U. Lemke, PhD
Part 1 from the last issue is available here.
Part 2 from the last issue is available here.
Part 3 from the last issue is available here.
Part 4 from the last issue is available here.
Part 5: Conclusion
5.1 Observations and questions
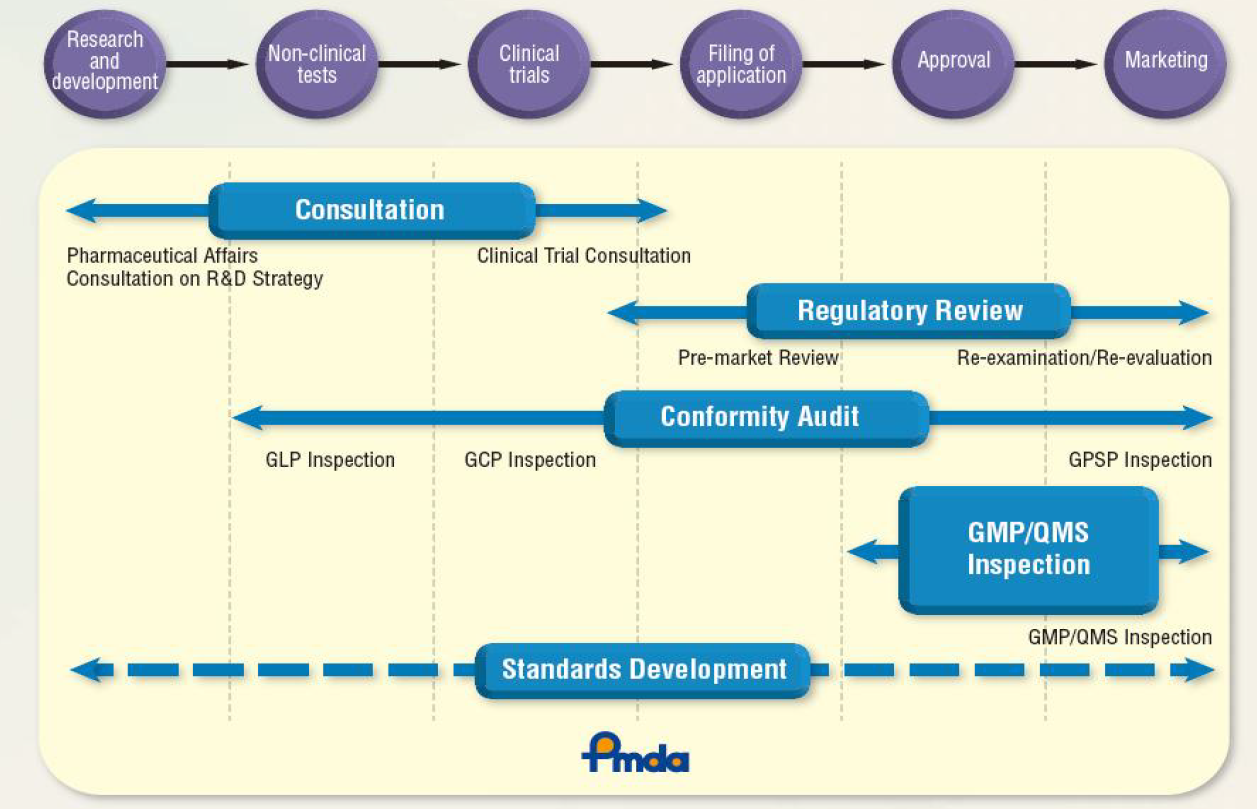
A significant number of functionalities in the Operating Room require (real-time) exchange of data and control information. Based on the IT architectures discussed above and generic standard issues outlined in a Weissbuch on “Sichere Dynamische Vernetzung in Operationssaal und Klinik” [13], these functionalities may best be understood by means of clinical scenarios or use cases which address real clinical requirements for interoperability. Approval of devices and systems which claim to have features to support such interoperability should be based on tests which include compliance to standards. This, however, poses a number of questions which need to be addressed in the development of the DOR, some of these are:
- 1. What functionality/feature changes to an already approved device distinguishes a predicate device 510(k) procedure from a new or post-predicate device (for example, augmented with new interoperability features), i.e. when and when not is a device substantially equivalent to a predicate device and may need to be classified as Class 3 requiring something similar to a Premarket Approval?
- 2. How will specific software (including for example, new Apps) for “intelligent” or web-enabled interoperability be classified in Japan, USA or Europe (taken into account the differences in device classification systems)?
- 3. What strategic steps in national and international approval organizations and technical and legal developments are necessary to raise the importance of IHE Connectathon and certification, as a basis for safety assessment in the approval process?
- 4. What strategic steps in national and international approval organizations and technical and legal developments are necessary to raise the importance of a scientific approach, as a basis for safety assessment in the approval process?
Another interesting observation relates to the classification of medical devices, which perhaps will become a major issue comparing FDA, PMDA and corresponding EU Directives. The latter states [20]:
“Where a Member State considers that the classification rules set out in Annex IX require adaptation in the light of technical progress and any information which becomes available under the information system provided for in Article 10, it may submit a duly substantiated request to the Commission and ask it to take the necessary measures for adaptation of classification rules. The measures designed to amend non-essential elements of this Directive relating to adaptation of classification rules shall be adopted in accordance with the regulatory procedure with scrutiny referred to in Article 7(3).”
The question here to be addressed in the future relates to whether devices augmented with (intelligent) software for interoperability qualify for the label “technical progress” and may therefore require, for an appropriate classification, an adaptation of the classification rules as given by the regulatory agencies. It remains to be seen, whether the new drafts for Amendments of the EU Directives concerning medical devices or the expected new PMDA regulations will take account of these new technological challenges.
5.2 Recommendations
It can be expected, that the complexity of the clinical and non-clinical tests for safety is very high and a solid scientific foundation [16] is necessary to show that a safe interoperability has been achieved. The PMDA drive to promote regulatory sciences is important in this context and may be of particular significance when devices and systems are planned to be employed in an international environment. From this and the observations made above, a number of recommendations can be derived:
- 1. In the middle or long term a Centre for interoperability in the OR with a strong focus on scientific methods and tools may have to be established on an international level, not least to establish completeness and reproducibility of testing procedures for clinical and non-clinical tests for interoperability of medical devices and systems for the OR, thereby enabling a higher confidence level for safety of medical device software and systems in the OR [19].
- 2. As can be expected that the role of IHE integration profiles will increase in importance for approval agencies in the future, IHE generally and IHE Surgery Connectathons specifically, can be considered to be the first steps in this direction and should become a focus of OR.NET, MD PnP and similar (follow-up) projects in the near future.
- 3. Leading industry for integrated ORs should be encouraged to take an active role in promoting activities towards recommendations 1 and 2. This does imply In particular, taking steps towards the definition of a set of promising IHE integration profiles which may then provide the basis for work items in the appropriate IHE domains.
- 4. A regular annual international OR interoperability forum for the exchange of views, concepts, R&D results, clinical and non-clinical safety testing, classification standards to facilitate conformity and predicate device testing, technical documentation, quality assessment and control of notified bodies, regulatory developments, etc. should be established. This forum should be of particular interest to SMEs engaging in the development of medical devices, in order to obtain a better understanding of resources required to achieve medical device approval on a national and international level. The CARS 2014 Workshop on “IHE Integration Profiles, Implementation and Approval Issues” [21] may serve as a template for such a forum.
Acknowledgement
The lecture contributions to the CARS 2014 workshops relating to IHE and approval issues, etc. by Prof. Ichiro Sakuma, the University of Tokyo, Japan and Prof. Kevin Cleary, the Children Medical Centre, Washington DC, USA as well as discussion contributions by over 20 international participants have been very much appreciated.
References
[13] Moser H. et al (2014) Weissbuch on “Sichere Dynamische Vernetzung in Operationssaal und Klinik, VDE, Frankfurt, Germany[16] Pace DK, Modelling and Simulation V&V Challenges, Johns Hopkins APL Technical Digest,
Vol.25, Number2 (2004) 163-172
[19] Lee I, et al., High-Confidence Medical Device Software and Systems. Published by IEEE Computer Society (April 2006).
[20] Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007 [21] http://www.cars-int.org
Heinz U. Lemke, PhD is a professor of Computer Science at the Technical University of Berlin since 1974, where he teaches and supervises research on the theme of Technical Informatics in Biomedicine. Since 1983 Heinz Lemke is the organizer of the congress series Computer Assisted Radiology and Surgery (CARS), editor-in-chief of the CARS Proceedings of the International Journal of CARS and executive director of the International Foundation of CARS.

Leave a Reply